Abstract
Background: Marginal zone lymphoma (MZL) is a group of indolent B-cell lymphomas that accounts for ~10% of non-Hodgkin's lymphomas (NHLs) in the US. Ibrutinib (ibr) is a first-in-class, once-daily inhibitor of Bruton's tyrosine kinase (BTK), which is the only US FDA approved therapy for patients (pts) with MZL who require systemic therapy and have received ≥1 prior anti-CD20-based therapy. A first-in-human study of ibr in various B-cell malignancies demonstrated that a fixed continuous dose of 560 mg was well-tolerated and led to full target occupancy; this dose was, therefore, selected for phase 2 studies (Advani, JCO 2013). This phase 2 study of single-agent ibr in pts with relapsed/refractory (R/R) MZL reported (median follow-up of 19.4 mo) an overall response rate (ORR) of 48%, clinical benefit rate (CBR) of 83%, median progression-free survival (PFS) of 14.2 mo with median overall survival (OS) and duration of response (DOR) not reached (NR), and median time to initial response of 4.5 mo. ORRs did not significantly differ between MZL subtypes (splenic, 54%; nodal, 41%; extranodal, 50%; Noy, Blood 2017). Ibr was also found to be safe and well-tolerated by these pts with R/R MZL. Here, we report additional analyses of clinical outcomes by prior therapy, MZL subtype, as well as baseline mutation profiling.
Methods: This phase 2 study enrolled pts with R/R MZL receiving ≥1 prior treatment for MZL including ≥1 CD20-containing regimen. Pts received oral ibr 560 mg once daily for up to 3 years or until disease progression or unacceptable toxicity. The primary endpoint was ORR (by IRC), and secondary endpoints included DOR, PFS, and OS. We report clinical outcomes for 60 IRC-evaluable pts based on prior therapy (rituximab only [RTX] or RTX-based chemoimmunotherapy [RTX-CIT]) and MZL subtype. A cancer specific targeted gene panel was used to perform mutation profiling on baseline tumor samples. Correlations of gene mutations with known clinical relevance in MZL to DOR, PFS, OS, and maximum change from baseline in sum of product diameters (SPD) were analyzed.
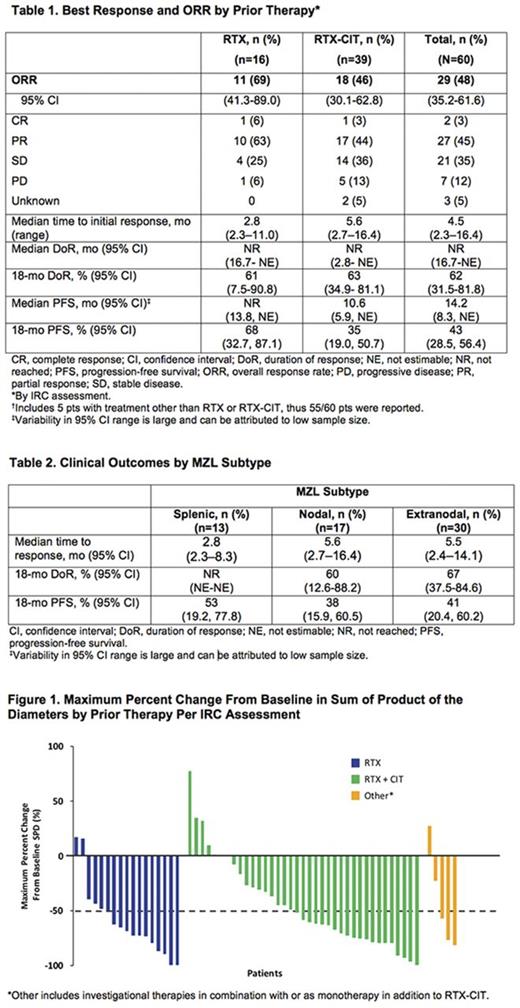
Results: Median age of the 63 enrolled pts with R/R MZL was 66 y (range, 30-92). Pts had a median of 2 prior therapies (range, 1-9). For the 16 (25%) pts treated with prior RTX, median number of prior therapies was 1 (range, 1-4); 39 (62%) pts who received prior RTX-CIT had median 2 prior therapies (range 1-9). Of 14 pts refractory to their last prior therapy at enrollment, 8 (57%) were in the prior RTX group compared with 4 (29%) in the prior RTX-CIT group. Response rates for the 60 evaluable pts by prior therapy are summarized in Table 1. ORR for the prior RTX and RTX-CIT groups were 69% and 46%, respectively. The response rates for the prior RTX vs. prior RTX-CIT groups were CR: 6% vs 3%; PR: 63% vs 44%; SD: 25% vs 36%; and PD: 6% vs 13% with a CBR of 94% vs 82%. A waterfall plot of maximum change in tumor burden by prior therapy is presented in the Figure (median change: RTX, −69%; RTX-CIT, −59%). The median time to initial response was 2.8 mo for prior RTX and 5.6 mo for prior RTX-CIT. Median DOR was not reached in either subgroup; the estimated 18-mo DOR rate was 61% for prior RTX and 63% for prior RTX-CIT. The estimated18-mo PFS rate was 68% for prior RTX, and 35% for prior RTX-CIT. The estimated 18-mo OS was 86% for prior RTX, and 82% for prior RTX-CIT. The time to initial response, 18-mo DOR, and 18-mo PFS for pts by MZL subtypes are shown in Table 2.
Baseline gene mutation profiling of tumor tissues from 41 pts showed that mutations in the BCR-NFkB signaling pathway, MYD88 and TNFAIP3 (A20), positively correlated with response (MYD88 with PFS and TNFAIP3 with SPD). Mutations in NOTCH2 and KMT2D (MLL2), which are not involved in BCR-NFkB signaling, were negatively correlated with DOR.
Conclusions: Single-agent ibr showed a trend toward higher ORR and median PFS, and a faster response in R/R MZL pts who previously received RTX when compared with those who received RTX-CIT. These results could be influenced by the characteristics of pts in each subgroup. In all cases, continued treatment with ibr resulted in increased clinical benefit. Mutations in the BCR-NFkB signaling pathway, (MYD88 and TNFAIP3), were positively correlated with response and mutations in NOTCH2 and KMT2D were negatively correlated with response. These results provide evidence of the efficacy of single-agent ibr in pts previously exposed to RTX monotherapy and support the use of ibr as a chemo-free option for MZL.
Chen: Merck: Consultancy, Other: travel expenses ; Genentech/Roche: Consultancy, Honoraria, Other: travel expenses, Speakers Bureau; Seattle Genetics: Consultancy, Honoraria, Other: travel expenses, Research Funding, Speakers Bureau; Pharmacyclics LLC, an AbbVie Company: Research Funding; Millennium: Honoraria, Research Funding, Speakers Bureau. Thieblemont: Roche: Consultancy, Honoraria, Research Funding; Janssen: Consultancy; Bayer: Consultancy, Honoraria; Celgene: Consultancy, Honoraria; Abbvie: Consultancy, Honoraria; Cellectis: Speakers Bureau; Sanofi: Other: travel expenses. Martin: Teva: Research Funding; Janssen: Consultancy, Honoraria, Other: travel expenses; Celgene: Consultancy; Genentech: Consultancy; Novartis: Consultancy; Gilead: Consultancy, Other: travel expenses. Flowers: National Institutes Of Health: Research Funding; Spectrum: Consultancy; Bayer: Consultancy; OptumRx: Consultancy; Prime Oncology: Research Funding; V Foundation: Research Funding; Clinical Care Options: Research Funding; Gilead: Consultancy; Eastern Cooperative Oncology Group: Research Funding; Janssen Pharmaceutical: Research Funding; Research to Practice: Research Funding; Celgene: Consultancy, Research Funding; Millennium/Takeda: Research Funding; Genentech/Roche: Consultancy, Research Funding; Onyx: Research Funding; National Cancer Institute: Research Funding; Acerta: Research Funding; Abbvie: Consultancy, Research Funding; TG Therapeutics: Research Funding; Burroughs Welcome Fund: Research Funding; Educational Concepts: Research Funding; Infinity: Research Funding; Pharmacyclics LLC, an AbbVie Company: Research Funding; Seattle Genetics: Consultancy. Morschhauser: Celgene: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Servier: Consultancy; Bristol-Myers Squibb: Consultancy, Honoraria; Gilead: Consultancy; Roche: Consultancy, Honoraria. Collins: Celleron: Consultancy; Roche: Consultancy, Honoraria, Speakers Bureau; Bristol-Myers Squibb: Research Funding; ADC Therapeutics: Research Funding; Takeda: Consultancy, Honoraria, Speakers Bureau; MSD: Consultancy, Honoraria; Amgen: Research Funding; Celgene: Research Funding; Pfizer: Consultancy, Honoraria. Ma: Novartis: Consultancy, Research Funding; Genentech: Consultancy, Speakers Bureau; AbbVie: Consultancy, Research Funding; Pharmacyclics LLC, an AbbVie Company: Consultancy, Research Funding, Speakers Bureau; Acerta: Research Funding; Gilead: Consultancy, Research Funding, Speakers Bureau. Coleman: Gilead: Consultancy, Research Funding, Speakers Bureau; Merck: Research Funding; AbbVie: Research Funding; Pharmacyclics LLC, an AbbVie Company: Research Funding, Speakers Bureau; Celgene: Consultancy, Research Funding, Speakers Bureau; Millenium: Research Funding; Janssen Oncology: Research Funding. Peles: Onyx: Honoraria, Other: travel expenses, Speakers Bureau; Novartis: Honoraria, Other: travel expenses, Speakers Bureau; Celgene: Honoraria, Other: travel expenses, Speakers Bureau; Takeda: Consultancy, Honoraria, Other: travel expenses, Speakers Bureau; SGN: Honoraria, Other: travel expenses, Speakers Bureau; Florida Cancer Specialists: Employment, Equity Ownership. Smith: Sharp: Research Funding; Seattle Genetics: Research Funding; Portola Pharmaceuticals: Research Funding; Merck: Research Funding; Acerta: Research Funding; Genentech: Research Funding; Pharmacyclics LLC, an AbbVie Company: Research Funding; Dohme Corp: Research Funding; Janssen: Research Funding. Barrientos: Gilead: Consultancy, Research Funding; Janssen: Consultancy; Pharmacyclics LLC, an AbbVie Company: Consultancy, Research Funding; AbbVie: Consultancy, Research Funding. Smith: AbbVie: Equity Ownership; Pharmacyclics LLC, an AbbVie Company: Employment, Other: travel expenses. Munneke: AbbVie: Equity Ownership; Pharmacyclics LLC, an AbbVie Company: Employment, Other: travel expenses. Wu: Pharmacyclics LLC, an AbbVie Company: Employment, Patents & Royalties; AbbVie: Equity Ownership. Cheung: AbbVie: Equity Ownership; Pharmacyclics LLC, an AbbVie Company: Employment, Patents & Royalties; Eli Lilly: Equity Ownership. Kwei: Pharmacyclics LLC, an AbbVie Company: Employment; AbbVie: Equity Ownership; Gilead Sciences: Employment, Equity Ownership. Samakoglu: Pharmacyclics LLC, an AbbVie Company: Employment. Arango-Hisijara: Pharmacyclics LLC, an AbbVie Company: Employment; AbbVie: Equity Ownership. Dimery: AbbVie: Equity Ownership; Pharmacyclics LLC, an AbbVie Company: Employment. Noy: Pharmacyclics LLC, an AbbVie Company: Honoraria, Other: Travel, Accommodation, Expenses, Research Funding, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal